Step-by-step explanation:
Ions are the species which form when a neutral atom either loses or gain an electron.
Atomic number of sodium is 11 and it is able to lose only one electron to attain stability. Hence, it will always form
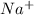 ion.
ion.
Atomic number of chlorine is 17 and being a non-metal it needs an electron to completely fill its octet. Hence, a neutral atom of Cl by gaining an electron change into
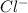 ion.
ion.
Atomic number of calcium is 20 and it needs to lose two electrons to become stable in nature. Hence, it will become a
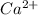 ion.
ion.
Atomic number of bromine is 35 and it being a non-metal it needs an electron to completely fill its octet. Hence, a neutral atom of Br by gaining an electron change into
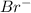 ion.
ion.
Atomic number of neon is 10 and it has completely filled octet. So, it neither needs to gain or lose an electron.
Hence, neon atom will remain neutral in nature.
Thus, we can conclude that out of the given options only
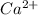 and
and
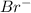 ions would be likely to form.
ions would be likely to form.