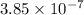Answer: The hydroxide concentration of this sample is
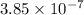
Step-by-step explanation:
When an expression is formed by taking the product of concentration of ions raised to the power of their stoichiometric coefficients in the solution of a salt is known as ionic product.
The ionic product for water is written as:
![K_w=[H^+]* [OH^-]](https://img.qammunity.org/2022/formulas/chemistry/college/utir0m14n2zmcpnotnmtin2nms2vsmda1z.png)
![7.7* 10^(-14)=[H^+]* [OH^-]](https://img.qammunity.org/2022/formulas/chemistry/college/4k6zkx86rax1hqgh0me46czksvqiq1e0et.png)
As
![[H^+]=[OH^-]](https://img.qammunity.org/2022/formulas/chemistry/college/cvc9f5zecwj2sa3gm3vvmzgkvh2p1qdz8n.png)
![2[OH^-]=7.7* 10^(-14)](https://img.qammunity.org/2022/formulas/chemistry/college/vdztxnyjdnnmx4wc3ov0uoktsa420l82sg.png)
![[OH^-]=3.85* 10^(-7)](https://img.qammunity.org/2022/formulas/chemistry/college/e0k8oxxs9qwby7h2yojeusxv8hj6x8ke3l.png)
Thus hydroxide concentration of this sample is