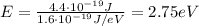The energy of a photon is given by
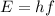
where h is the Planck constant and f is the photon frequency.
We can find the photon's frequency by using the following relationship:
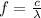
where c is the speed of light and
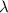
is the photon's wavelength. By plugging numbers into the equation, we find

And so now we can find the photon energy
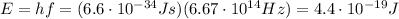
We know that 1 Joule corresponds to
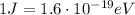
So we can convert the photon's energy into electronvolts: