Answer: Option (D) is the correct answer.
Step-by-step explanation:
It is known that metals are the substances which hold excess number of electrons and hence they need to lose electrons in order to gain stability.
For example, potassium is a metal with atomic number 19 and its electronic configuration is 2, 8, 8, 1.
So, it needs to gain stability and hence, it easily loses its one valence electron to acquire a positive charge as
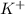 .
.
Thus, we can conclude that in general, metals react by losing valence electrons.