Answer:
Grams of Ti = 4.56 g
Step-by-step explanation:
Given:
# atoms of Titanium = 5.74*10²²
To determine:
The mass of Ti in corresponding to the given number of atoms
Step-by-step explanation:
1 mole of any substance contains Avogadro's number of atoms.
i.e. 1 mole of Ti = 6.023*10²³ atoms
Since 1 mole Ti = 47.90g,
47.90 g of Ti contains 6.023*10²³ atoms
Therefore,
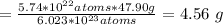 of Ti would correspond to:
of Ti would correspond to: