Answer:
11 molecules of CH4.
23 atoms of C is the leftover.
Step-by-step explanation:
Hello!
In this case, for the formation of methane:
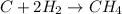
We can see there is an excess of carbon based on their stoichiometry, because the needed amount of hydrogen gas molecules would be:
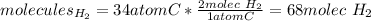
Thus, the formed molecules of methane are computed below:
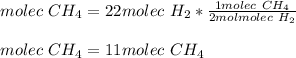
In such a way, the leftover of carbon atoms are:
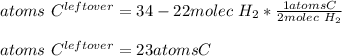
Best regards!