Answer:
KCl
Step-by-step explanation:
Hello,
Potassium chloride is well-known as an ionic compound owing to the shown-below greater-than-1.7 difference between the electronegativities of potassium and chlorine:
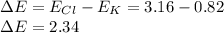
This is by cause of the fact that such compounds that have a difference in the constituent elements' electronegativities greater than 1.7 are considered as ionic.
Best regards.