Answer:
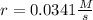
Step-by-step explanation:
Hello!
In this case, considering the given table, we are able to represent the symbolic rate law as shown below:
![r=k[BF_3]^m[NH_3]^n](https://img.qammunity.org/2022/formulas/chemistry/college/b9zvji1mk3y5agq32wsf5p0m0e1b5anqhf.png)
Thus, by using the following steps, we can find both m (BF3 order of reaction) and n (BF3 order of reaction):
- Experiment 1 and 2 for the calculation of n:
![(r_1)/(r_2)=(k[BF_3]_1^m[NH_3]_1^n)/(k[BF_3]_2^m[NH_3]_2^n)](https://img.qammunity.org/2022/formulas/chemistry/college/cct25l2tut21dd7q5zffbmlyocasd6fv7f.png)
So we plug in to obtain:
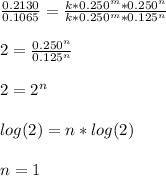
So the order of reaction with respect to NH3 is 1.
- Experiment 3 and 4 for the calculation of m
![(r_3)/(r_4)=(k[BF_3]_3^m[NH_3]_3^n)/(k[BF_3]_4^m[NH_3]_4^n)](https://img.qammunity.org/2022/formulas/chemistry/college/4huji3zfmfibidl4geh73snnsd7x5sy4dt.png)
So we plug in to obtain:
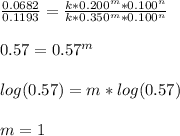
So the order of reaction with respect to BF3 is also 1.
Now, we can compute the rate constant by solving for it on any of the experiments there, say experiment 1:
![k=(r_1)/([BF_3][NH_3]) =(0.2130M/s)/(0.250M*0.250M)\\\\k=3.41M^(-1)s^(-1)](https://img.qammunity.org/2022/formulas/chemistry/college/yk21vc8x8ghp206n6sb6hi4ywagdp9jk6l.png)
Thus, the initial reaction rate for the 0.50M BF3 and 0.020M NH3 is:
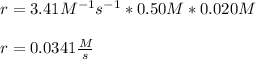
Best regards!