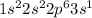Answer: The correct answer is
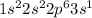
Step-by-step explanation:
Electronic configuration is defined as the configuration in which total number of electrons are represented which are present in an element.
Number of electrons are determined by the atomic number of that element.
Atomic number of Sodium = 11
Electronic configuration of sodium element (Na) :
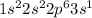
Hence, the correct answer is