Answer:
Option c
Step-by-step explanation:
Atomic number of helium is 2.
Electronic configuration of He =
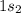
First energy shell of an atom is known as K shell. It has only one sub shell 's sub shell'.
K shell or energy level can accommodate maximum of 2 electrons. Helium has 2 electrons and these two electrons are filled in K shell. So, atom of helium has a full energy level.
Elements having full energy level or eight electrons in their valence shell is stable and therefore chemically inactive or inert.
As outer shell of helium is full, so it is chemically inactive.