Answer: Option (A) is the correct answer.
Step-by-step explanation:
When two compounds react together then certain amount of energy must be supplied in order to form new substance. But when heat supplied is less than the heat absorbed by the reactants then there will be very less release of energy.
The reaction between ammonium chloride and water will be as follows.
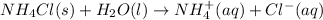
Therefore, when we dissolve ammonium chloride in to water then ammonium chloride will absorb heat and dissociate into ions and the solution becomes colder. Thus, the reaction will be endothermic in nature because heat is absorbed by the reactants.
Hence, we can conclude that endothermic reaction is occurring when ammonium chloride is dissolved in water.