The frequency of the
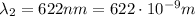
wavelength photon is given by
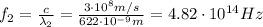
where c is the speed of light.
The energy of this photon is
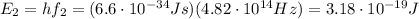
where h is the Planck constant.
The energy of the first photon is twice that of the second photon, so
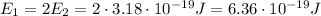
And so now by using again the relationship betwen energy and frequency, we can find the frequency of the first photon:
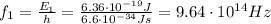
and its wavelength is
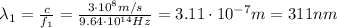
So, we see that the wavelength of the first photon is exactly half of the wavelength of the second photon (622 nm).