Step-by-step explanation:
The given equation is as follows.
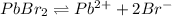
s 2s
It is given that,
![K_(sp) = [Pb^(2+)][Br^(-)]^(2) = 6.60 * 10^(-6)](https://img.qammunity.org/2019/formulas/chemistry/college/4xxbj4c7u61g87g5kt6nq2lnq1aawbvdos.png)
Let the solubility of given ions be "s".
Since, KBr on dissociation will given bromine ions.
Hence,
![K_(sp) = [Pb^(2+)] * ([Br^(-)])^(2)](https://img.qammunity.org/2019/formulas/chemistry/college/xt2m5u3frc861x6k3iae76a9lp98dteexh.png)
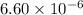 =
=
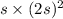
=
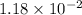 M
M
Therefore, solubility of
![[PbBr_(2)]](https://img.qammunity.org/2019/formulas/chemistry/college/75k8641mxv8b9pto87x1d6mtl3ifq1te0d.png) is
is
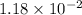 M in KBr.
M in KBr.
Now, we will calculate the molar solubility of
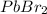 in 0.5 M KBr solution as follows.
in 0.5 M KBr solution as follows.
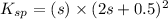
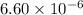 =
=
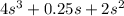
s =
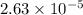
Thus, we can conclude that molar solubility of
![[PbBr_(2)]](https://img.qammunity.org/2019/formulas/chemistry/college/75k8641mxv8b9pto87x1d6mtl3ifq1te0d.png) in 0.500 m KBr solution is
in 0.500 m KBr solution is
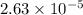 .
.