1) Number of moles = (weight of solute in grams)/ (gram molecular weight)
For NaCl, gram molecular weight = 58.5
∴ Number of moles of NaCl = 24 / 58.5 = 0.408
2) 1 ml = 10^-3 liters
∴ 475 ml = 475 X 10^-3 liters = 0.475 liter
3) Molarity of solution =
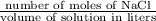
=

= 0.8589 M