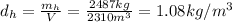Missing question: "what is the density of the hot air?"
Solution:
The balloon is floating at constant height, and this means that the two forces acting on it (the weight and the buoyant force) are in equilibrium:
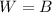
where W is the weight of the balloon, which is sum of the weight of the balloon structure and of the hot air inside the balloon, and B is the buoyant force.
The buoyant force is given by:
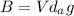
where V is the volume of the balloon, d is the air density and g is the gravitational acceleration. Plugging numbers into the equation, we find
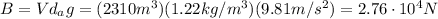
This is equal to the weight of the balloon+hot air inside it:
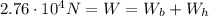
where
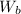
is the weight of the balloon
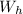
is the weight of the hot air inside the balloon
The weight of the balloon is
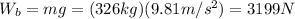
Which means that the weight of the hot air is
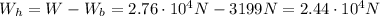
which corresponds to a mass of
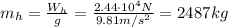
And since the mass is the product between density and volume, we can find the density of the hot air: