whatever% of anything is just (whatever/100) * anything.
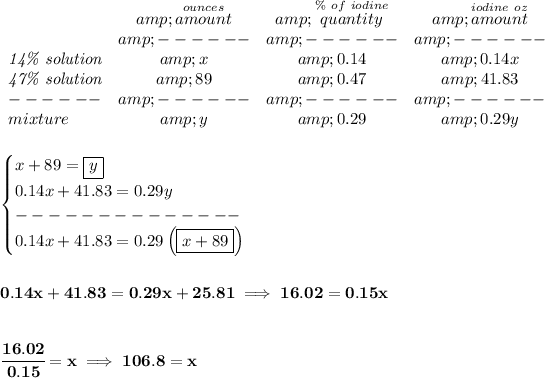
x = ounces of 14% iodine.
y = ounces of 29% iodine.
we know that in a 14% iodine solution, 14% is iodine and the rest is something else, we also know that "x" ounces of the solution have 14% of iodine, how much is that? (14/100) * x, or 0.14x.
likewise, in the 89 ounces of 47% solution there is (47/100) * 89 of iodine.
and likewise as well, in "y" ounces of 29% iodine there is (29/100) * y, or 0.29y of iodine.
bearing in mind that, whatever "x" and "y" may be, x + 89 = y, and that the sum of the iodine amounts also will equate 0.29y.