Answer : The correct answer for the Theoretical Yield is 48.93 g of product .
Theoretical yield : It is amount of product produced by limiting reagent . It is smallest product yield of product formed .
Following are the steps to find theoretical yield .
Step 1) : Write a balanced reaction between Al and Cl₂ .
2 Al + 3 Cl₂→ 2 AlCl₃
Step 2: To find amount of product (AlCl₃) formed by Al .
Following are the sub steps to calculate amount of AlCl₃ formed :
a) To calculate mole of Al :
Given : Mass of Al = 35.5 g
Mole can be calculate by following formula :
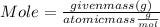
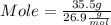
Mole = 1.32 mol
b) To find mole ratio of AlCl₃ : Al
Mole ratio is calculated from balanced reaction .
Mole of Al in balanced reaction = 2
Mole of AlCl₃ in balanced reaction = 2.
Hence mole ratio of AlC; l₃ : Al = 2:2
c) To find mole of AlCl₃ formed :
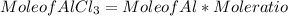
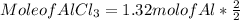
Mole of AlCl₃ = 1.32 mol
d) To find mass of AlCl₃
Molar mass of AlCl₃ =
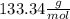
Mass of AlCl3 can be calculated using mole formula as:
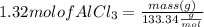
Multiplying both side by
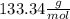
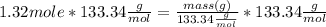
Mass of AlCl₃ = 176.00 g
Hence mass of AlCl₃ produced by Al is 176.00 g
Step 3) To find mass of product (AlCl₃) formed by Cl₂ :
Same steps will be followed to calculate mass of AlCl₃
a) Find mole of Cl₂
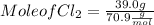
Mole of Cl₂ = 0.55 mol
b) Mole ratio of Cl₂ : AlCl₃
Mole of Cl₂ in balanced reaction = 3
Mole of AlCl₃ in balanced reaction = 2
Hence mole ratio of AlCl₃ : Cl₂ = 2 : 3
c) To find mole of AlCl₃
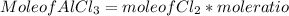
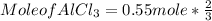
Mole of AlCl3 = 0.367 mol
d) To find mass of AlCl₃ :
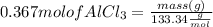
Multiplying both side by
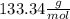
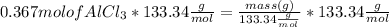
Mass of AlCl₃ = 48.93 g
Hence mass of AlCl₃ produced by Cl₂ = 48.93 g
Step 4) To identify limiting reagent and theoretical yield :
Limiting reagent is the reactant which is totally consumed when the reaction is complete . It is identified as the reactant which produces least yield or theoretical yield of product .
The product AlCl₃ formed by Al = 176.00 g
The product AlCl₃ formed by Cl₂ = 48.93 g
Since Cl₂ is producing less amount of product hence it is limiting reagent and 48.93 g will be considered as Theoretical yield .