Take the molar mass of each element within the compound.
1 mole of the following...
K = 39g
Cl = 35g
O = 16g
Now find the molar mass of the compound itself.
K = 39
Cl = 35
O₃ = 16 * 3 = 48
39+35+48 = 122g
Set up a proportion with the amount of KClO₃ used, the molar mass of KClO₃, the amount of oxygen taken from a mole of KClO₃, and the amount of oxygen you will take from the amount of KClO₃ you have.
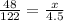
Cross multiply to get the following equation:
122x = 216
Divide both sides by 122 to solve for x.
x = 1.77
You can prepare
1.77g of O₂ from 4.5g of KClO₃.