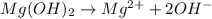Answer : The correct option is,
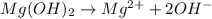
Explanation :
Ionization : It is a type of process in which the molecule obtain a negative and a positive charge by losing or gaining the electrons.
(1)
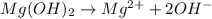
This reaction is an example of ionization in which the magnesium hydroxide ionizes into magnesium (2+) ion and hydroxide ion.
(2)
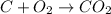
This reaction is not an example of ionization reaction. This reaction is an example of synthesis reaction in which the two or more reactants that are present in their elemental state combine to give a new product.
(3)
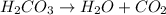
This reaction is not an example of ionization reaction. This reaction is an example of decomposition reaction in which the larger reactant molecule decomposes to give two or more smaller products.
(4)
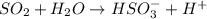
This reaction is not an example of ionization reaction.
Hence, the correct option is,