Answer:
Al
Step-by-step explanation:
The quantities in grams of each reactants are
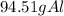
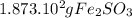
The grams of
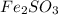 are expressed in scientific notation
are expressed in scientific notation
Since the power of ten is positive and is iqual 2 we must run the comma two spaces to the right
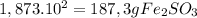
Quantities of reactans expressed without scientific notation
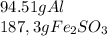
The amount in grams of Al is less than the amount of
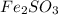 therefore it will be finished first.
therefore it will be finished first.