Answer:The correct answer is option b.
Step-by-step explanation:
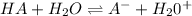
The equilibrium constant expression is given as:
![K_(eq)=([A^-][H_3O^+])/([HA][H_2O])](https://img.qammunity.org/2019/formulas/chemistry/high-school/efff45256ugn5bl89chqqcs81w7zzdyznw.png)
![K_a=K_(eq)* [H_2O]=([A^-][H_3O^+])/([HA])](https://img.qammunity.org/2019/formulas/chemistry/high-school/l7m0urn22l6oiswtb61hr6qk2i8xmsi3m4.png)
The acid dissociation constant is product of equilibrium constant and concentration of water.
Hence,from the given options the correct answer is option b.