Answer:
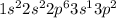 .
.
Step-by-step explanation:
Electronic configuration is defined as the distribution of electrons in the energy levels around an atomic nucleus.
Atomic number helps in determining the electronic distribution of an atom. We write electronic configuration according to Aufbau's principle in which shells are arranged in increasing energy levels.
Aluminium (Al) is a p block element and its atomic number is 13.
Electronic configuration of aluminium is
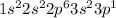 .
.
Excite state is achieved when the electron moves to a higher energy level.The 3s electron moves to 3p orbital so that it can form three bonds.
Thus Electronic configuration of aluminium in an excited state is
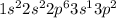 .
.