Let's assume that the gas is an ideal gas.
1 mole of ideal gas has the volume as 22.4 L at STP (273 K , 1 atm)
The given gas has 10.00 L. Hence we can find out the moles of gas.
moles in 22.4 L gas = 1 mol
moles in 10.00 L gas =
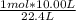
= 0.446 mol
moles = mass / molar mass
hence, molar mass = mass / moles
= 11.92 g / 0.446
= 26.73 g/mol
Hence, the molar mass of the gas is
26.73 g/mol