Step-by-step explanation:
1) Pentyne is an unsaturated hydrocarbon with formula
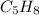 . It is an alkyne molecule with single triple bond present in it.
. It is an alkyne molecule with single triple bond present in it.
Correct statements are:
- Contains 5 carbons
- Has a triple bond
- Is an alkyne
2) Alkenes are unsaturated hydrocarbons with double bonds.
- They are unsaturated.
- They are reactive.
- They have double bonds.
3) Butene is an unsaturated hydrocarbon with 4 carbon atoms and double bonds.
4)Alkene undergo addition reaction easily.
5)If the carbon atom of hydrocarbon is bonded to less than 4 other atoms, the hydrocarbon is considered unsaturated.
6)The correct statements are:
- They have double and triple bonds.
- They will react readily with other elements and compounds.
7)The general formula of :
Alkane =
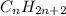
Alkene =
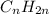
Alkyne =
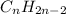
The
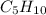 is an alkene with double C-C bond.
is an alkene with double C-C bond.
8) The general formula of alkene is given by:
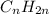
9) Benzene is an aromatic cyclic compound with six membered carbon ring with alternative double bonds. Due to conjugation of double bond all the C-C bonds are identical.
- All C-C bonds share 2 pairs of electrons.
- All of the C-C bonds are actually identical.
- The structure of the molecule is closed like a ring structure.