Answer:
The correct answer is d.
Step-by-step explanation:
Step 1: First we must obtain the molecular weight of
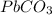 and Pb.
and Pb.
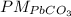 = 267,21
= 267,21
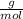
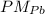 = 207,2
= 207,2
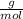
Step 2: We can now calculate the percentage of lead in a
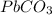 molecule by stating a simple rule of three:
molecule by stating a simple rule of three:
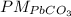 --> 100%.
--> 100%.
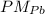 --> X
--> X
%Pb =
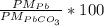
%Pb =
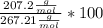
%Pb = 77,54 %
Have a nice day!