The final temperature is 45.45 Celcius
Step-by-step explanation:
The Combined Gas Law:
The combined gas law allows to derive relationships between the variable that undergoes like pressure, temperature and volume.
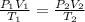
It is given thatpressure is constant so,
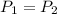
Hence combined gas law becomes,
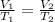
Substituting the values given in the question,
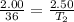
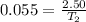
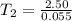
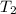 =45.45 C
=45.45 C