Answer:
Single and double
Step-by-step explanation:
Hello,
Benzene is an aromatic organic chemical compound with the following chemical formula:
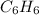 .
.
Benzene molecule make up six carbon atoms linked in a hexagoned ring with one hydrogen atom bonded to each carbon to attain the octate's rule. It is an hydrocarbon since it just has carbon and hydrogen.
Now, due to its arrangement shown on the attached picture, one states that it has interleaved single and double bonds.
Best regards.