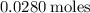Let's review what is given in this problem.
The volume of the solution is 11.2 milliliters, which is 0.0112 liters (multiply 11.2 mL with 1 L / 1000 mL to get 0.0112).
The molar concentration is 2.5 M. This means that there are 2.5 moles of NaOH per liter in the solution.
Multiply the volume and the molar concentration to get the moles of NaOH in the solution.
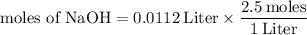
The "Liter" will cancel out, leaving only moles.
After using a calculator (or computing by hand), you should get the following value:
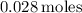
Since the given values were given with 3 significant figures, let's change this answer so there are 3 significant figures.
Thus, your final answer is