Answer:
C, F
Step-by-step explanation:
A Lewis structure represents only the valence electrons an atom has. That said, in this specific problem we're considering the valence electrons of an atom. We wish to have p-orbital electrons in the valence shell.
Let's look at the electron configurations of each atom including the number of valence electrons:
- Hydrogen only has one valence electron, as it's in group 1A. Its electron configuration is
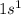 . It doesn't have any p electrons.
. It doesn't have any p electrons. - Carbon has 4 valence electrons (group 4A). Its electron configuration is
![[He]2s^2 2p^2](https://img.qammunity.org/2020/formulas/chemistry/middle-school/1groog7ytyaf5brgj3fpe03f55gyxgn8kd.png) . Notice that the valence shell contains two p electrons.
. Notice that the valence shell contains two p electrons. - Calcium has 2 valence electrons (group 2A). Its electron configuration is
![[Ar]4s^2](https://img.qammunity.org/2020/formulas/chemistry/middle-school/99w90qjuvs7ex8igfb7e19qs7mmnwo3izy.png) . It only has s electrons in its valence shell.
. It only has s electrons in its valence shell. - Fluorine has 7 valence electrouns (group 7A). Its electron configuration is
![[He]2s^2 2p^5](https://img.qammunity.org/2020/formulas/chemistry/middle-school/vzuyo86udz4tduejihe1xweyuau35sc9jj.png) . It has five p electrons in its valence shell.
. It has five p electrons in its valence shell.