Answer:
W = 918.18 J
Step-by-step explanation:
given,
Initial pressure of balloon = 815.0 Pa
Initial Volume = 0.7 m³
volume expand by = 5 times
From the ideal gas equation
PV = nRT
And work is equal to
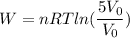
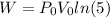
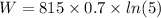
W = 918.18 J
Work done by expanding the balloon is equal to W = 918.18 J