Answer : The total number of carbons present in ethylheptane are, 9.
Explanation :
The basic rules for naming of organic compounds are :
First select the longest possible carbon chain.
For the number of carbon atom, we add prefix as 'meth' for 1, 'eth' for 2, 'prop' for 3, 'but' for 4, 'pent' for 5, 'hex' for 6, 'sept' for 7, 'oct' for 8, 'nona' for 9 and 'deca' for 10.
A suffix '-ane' is added at the end of the name of alkane.
If two of more similar alkyl groups are present, then the words 'di', 'tri' 'tetra' and so on are used to specify the number of times these alkyl groups appear in the chain.
The given compound is, ethylheptane.
In the given compound, the prefix used is 'hept' that means longest possible carbon chain number will be 7 and suffix '-ane' shows that the compound belongs to alkane group and the substituent 'ethyl'
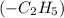 is also attached to the main carbon chain. Thus, the total number of carbons present in ethylheptane are, 9.
is also attached to the main carbon chain. Thus, the total number of carbons present in ethylheptane are, 9.
Hence, the total number of carbons present in ethylheptane are, 9.