Answer:
Energy,
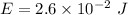
Step-by-step explanation:
It is given that,
Number of photons,
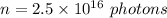
Wavelength,
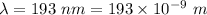
Let E is the energy delivered to the cornea per laser pulse. The energy of photon is terms of number of photon is given by :
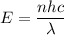
Where
h is Planck's constant
c is the speed of light
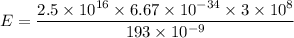
E = 0.0259 J
or
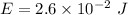
So, the energy delivered to the cornea per laser pulse is
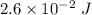 . Hence, this is the required solution.
. Hence, this is the required solution.