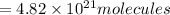Answer:
Step-by-step explanation:
Given
Volume of fixed chamber
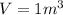
Initial Temperature
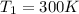
Final Temperature
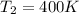
Heat Supplied
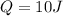
From First law of thermodynamics
Change in internal energy of the system is equal to heat added minus work done by the system
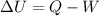
as the volume is fixed therefore work
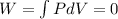
thus
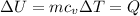
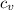 for mono-atomic gas is
for mono-atomic gas is
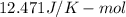
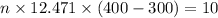
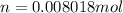
and 1 mole contains
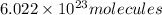
thus No of molecules
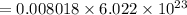
No of molecules