This is a incomplete question.The complete question is:
A chemist adds 180.0 ml of a 1.77 mol/L of sodium thiosulfate solution to a reaction flask. Calculate the mass in grams of sodium thiosulfate the chemist has added to the flask. Be sure your answer has the correct number of significant digits.
Answer: 50.4 g
Step-by-step explanation:
To calculate the number of moles for given molarity, we use the equation:
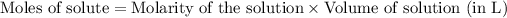 .....(1)
.....(1)
Molarity of sodium thiosulfate solution = 1.77 M
Volume of sodium thiosulfate solution = 180.0 mL = 0.1800 L
Putting values in equation 1, we get:
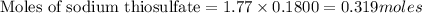
Mass of sodium thiosulfate =
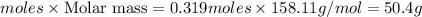
Thus 50.4 g of sodium thiosulfate the chemist has added to the flask.