Answer:
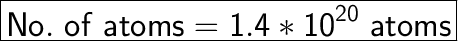
Step-by-step explanation:
Given Data:
Volume = v = 5000 cm³ = 0.005 m³
Density of CO₂ at RTP = 1.98 kg / m³
Molar Mass = M = 12 + 16 * 2 = 44 g / mol
Avogadro's No. =
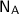 = 6.023 * 10²³ mol⁻¹
= 6.023 * 10²³ mol⁻¹
Required:
Number of atoms = ?
Solution:
We know that:
No. of moles (n) = mass in grams / molar mass ∴ Mass = Density * Volume
n = D * v / M
n = 1.98*0.005 / 44
n = 0.000225
Now, Finding the number of atoms
No. of atoms = No. of moles * Avogadro's Number
No. of atoms = 0.000225 * 6.023 * 10²³
No. of atoms = 0.0014 * 10²³
No. of atoms = 1.4 * 10²⁰ atoms
![\rule[225]{225}{2}](https://img.qammunity.org/2022/formulas/mathematics/high-school/3icqlwn6du2l5ygbr7z2lp6sjjralcpq09.png)
Hope this helped!
~AH1807