Answer : The energy of the photon emitted is, -12.1 eV
Explanation :
First we have to calculate the
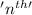 orbit of hydrogen atom.
orbit of hydrogen atom.
Formula used :
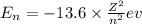
where,
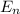 = energy of
= energy of
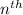 orbit
orbit
n = number of orbit
Z = atomic number of hydrogen atom = 1
Energy of n = 1 in an hydrogen atom:
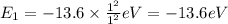
Energy of n = 2 in an hydrogen atom:
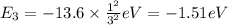
Energy change transition from n = 1 to n = 3 occurs.
Let energy change be E.
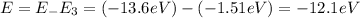
The negative sign indicates that energy of the photon emitted.
Thus, the energy of the photon emitted is, -12.1 eV