The question is incomplete, here is a complete question.
A solution is prepared at 25 °C that is initially 0.18 M in methylamine (CH₃NH₂), a weak base with
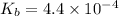 , and 0.35 M in methylammonium bromide (CH₃NH₃Br). Calculate the pH of the solution. Round your answer to 2 decimal places.
, and 0.35 M in methylammonium bromide (CH₃NH₃Br). Calculate the pH of the solution. Round your answer to 2 decimal places.
Answer : The pH of the solution is, 10.36
Explanation :
First we have to calculate the value of
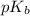 .
.
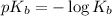
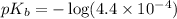
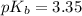
Now we have to calculate the value of pOH.
Using Henderson Hesselbach equation :
![pOH=pK_b+\log ([Salt])/([Base])](https://img.qammunity.org/2020/formulas/chemistry/college/c0pbb17evv0vtqf02zjej0e5fxdn212yx8.png)
![pOH=pK_b+\log ([CH_3NH_3Br])/([CH_3NH_2])](https://img.qammunity.org/2020/formulas/chemistry/college/m6ytgxomtbml2v8dhu0wqsvdjrcrtfu09x.png)
Now put all the given values in this equation, we get :
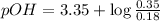
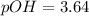
Now we have to calculate the pH.
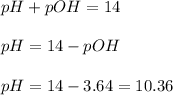
Therefore, the pH of the solution is, 10.36