Answer:
The balanced chemical reaction is
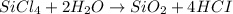
Explanation:
According to Law of conservation of mass, in an chemical reaction the mass of the reactant must be equal to the mass of the product obtained in the reaction.
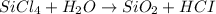
On the reactant side On the product side
Si = 1 Si = 1
Cl = 4 Cl = 1
H= 2 H= 2
O= 1 O= 2
Lets first balance Chlorine (Cl) by adding 4 on the product side
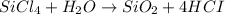
On the reactant side On the product side
Si = 1 Si = 1
Cl = 4 Cl = 4
H= 2 H= 4
O= 1 O= 2
Now lets balance H and O by adding 2 on the product side
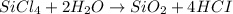
On the reactant side On the product side
Si = 1 Si = 1
Cl = 4 Cl = 4
H= 4 H= 4
O= 2 O= 2