Answer : The daughter nucleus is, polonium (Po).
Explanation :
Alpha decay : In this process, alpha particles is emitted when a heavier nuclei decays into lighter nuclei. The alpha particle released has a charge of +2 units.
The general representation of alpha decay reaction is:
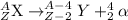
The complete alpha decay reaction will be,
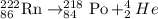
From this we conclude that, when radon-222 undergoes alpha decay then it transmutates into polonium daughter nucleus.
Hence, the daughter nucleus is, polonium (Po).