Answer:
21.344%
Step-by-step explanation:
For the given chemical reaction, 8 moles of the reactant should produce 4 moles of
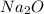 . However, 195 g of
. However, 195 g of
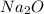 was produced instead. The molar mass of
was produced instead. The molar mass of
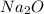 is 61.9789 g/mol.
is 61.9789 g/mol.
Thus, the moles of
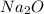 produced = 195/61.9789 = 3.1462 moles
produced = 195/61.9789 = 3.1462 moles
The percent error = [(Actual -Experiment)/Actual]*100%
The percent error = [(4.00 - 3.1462)/4.00]*100% = (0.85376/4.00)*100% = 21.344%