Answer : The correct option is, (d) 90 mL
Explanation :
First we have to calculate the volume of an object.
As we know that,

Given:
Density of an object = 0.25 g/mL
Mass of an object = 10 g
Now put all the given values in the above formula, we get:
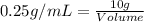
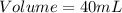
Thus, the volume of an object is 40 mL.
Now we have to calculate the height of the water in the graduated cylinder rise.
As we are given that:
The volume of water in graduated cylinder = 50 mL
The volume of an object = 40 mL
The height of the water in the graduated cylinder rise = Volume of water in graduated cylinder + Volume of an object
The height of the water in the graduated cylinder rise = 50 mL + 40 mL
The height of the water in the graduated cylinder rise = 90 mL
Therefore, the height of the water in the graduated cylinder rise will be, 90 mL