Answer:
The correct answer is option E.
Step-by-step explanation:
For wavelength to be minimum, energy would be maximum, i.e the electron will jump to infinite level.
Using Rydberg's Equation:
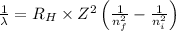
Where,
 = Wavelength of radiation
= Wavelength of radiation
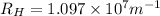 = Rydberg's Constant
= Rydberg's Constant
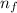 = Higher energy level
= Higher energy level
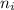 = Lower energy level
= Lower energy level
Z = atomic number
For hydrogen , Z = 1
The wavelength of light associated with the n = 2 to n = 1 electron transition in the hydrogen spectrum=
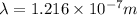
Putting the values, in above equation, we get
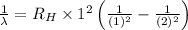
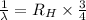 ..[1]
..[1]
For
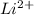 ,
,
Z = 3
The wavelength of light associated with the n = 2 to n = 1 electron transition in the lithium ion =
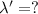
Putting the values, in above equation, we get
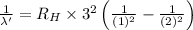
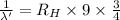 ..[2]
..[2]
Dividing [1] and [2]
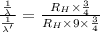
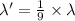
The coefficient required to be multiplied with wavelength of transition given for hydrogen spectrum to obtain the wavelength associated with the same electron transition in the
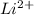 ion is 1/9.
ion is 1/9.