Given :
If I inhale 2.2 liters of gas at a temperature of 180 C and it heats to a temperature of 380 C in my lungs.
To Find :
The new volume of the gas.
Solution :
Since, their is no information about pressure, so we will assume that pressure is constant.
We know, relation between temperature and volume in constant pressure is :
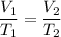
Putting all given values in above equation, we get :
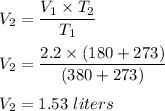
Hence, this is the required solution.