Answer:
See attached pictures.
Step-by-step explanation:
Hello,
On the attached pictures you will find the procedures regarding to each problem.
It is necessary to consider that the Hess Law states "that the change of enthalpy in a chemical reaction (i.e. the heat of reaction at constant pressure) is independent of the pathway between the initial and final states", in such a way, one modifies each sub-reaction to attain the main one, of course, the enthalpy of reaction must be inverted as the inverse reaction will account for the overall process. In some exercises, it was necessary to multiply the chemical reaction by a whole number to attain the main reaction, for instance, in the first exercise, the second subreaction was inverted and the first and the second multiplied by two and the third one by 3, therefore, both the stoichiometric coefficients and the given enthalpies of reaction are multiplied as well in order to compute the enthalpy of that reaction.
On the other hand, the enthalpy of reaction for the exercises 5 to 8 were computed as widely worked:
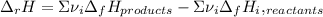
Whereas
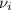 accounts for the stoichiometric coefficient of the ith compound based on the standard enthalpies of formation.
accounts for the stoichiometric coefficient of the ith compound based on the standard enthalpies of formation.
Best regards.