Answer:
1.312 x 10⁻¹² J/nucleon
Step-by-step explanation:
mass of ¹³⁶Ba = 135.905 amu
¹³⁶Ba contain 56 proton and 80 neutron
mass of proton = 1.00728 amu
mass of neutron = 1.00867 amu
mass of ¹³⁶Ba = 56 x 1.00728 amu + 80 x 1.00867 amu
= 137.10128 amu
mass defect = 137.10128 - 135.905
= 1.19628 amu
mass defect = 1.19628 x 1.66 x 10⁻²⁷ Kg
= 1.9858 x 10⁻²⁷ Kg
speed of light = 3 x 10⁸ m/s
binding energy,
E = mass defect x c²
E = 1.9858 x 10⁻²⁷ x (3 x 10⁸)²
E = 17.87 x 10⁻¹¹ J/atom
now,
binding energy per nucleon =
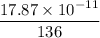
= 0.1312 x 10⁻¹¹ J/nucleon
= 1.312 x 10⁻¹² J/nucleon