Answer: The student added an extra 15 mL of distilled water to the
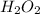 solution.
solution.
Explanation: As it is already mentioned that the student calculated the amount of moles of
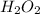 larger than the actual value in the titration of potassium permanganate solution, the most obvious and correct answer leading to this difference is the extra amount of
larger than the actual value in the titration of potassium permanganate solution, the most obvious and correct answer leading to this difference is the extra amount of
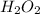 used during the analysis.
used during the analysis.
The student failed to wear goggles would come under the precautions while doing an experiment in the lab.
The student did not swirl the flask appropriately and therefore stopped short of the endpoint. This reason is the hypothetical one.