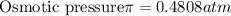Answer:
The osmotic pressure is 0.4808 atm
Step-by-step explanation:
Given:
Temperature =
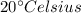
Volume of sucrose = 0.69 grams
volume of water = 100 mL
To Find:
The osmotic pressure =?
Solution:
Step 1: lets find the number of moles in 0.69 grams of sucrose(
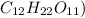
number of moles in 0.69 grams of sucrose =
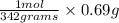
=>
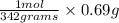
=>
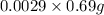
=>0.002 moles
Step2 :Dividing number of moles by the amount of litres in the solution
=>
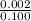 [ 100 mL = 0.100L]
[ 100 mL = 0.100L]
=> 0.02 M
Step 3: Converting the temperature to kelvin
=>20 +273
=>293 K
Step 4: Finding the value of osmotic pressure
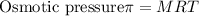
where
M is the Molarity
R is gas constant
T is the temperature
Now substituting the values,