Answer:
The answer to your question is 0.036 M
Step-by-step explanation:
Data
Molarity = ?
mass = 4.0 grams KOH
Volume = 2 L
Molecular mass KOH = 39 + 16 + 1 = 56 g
Formula
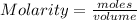
Process
1.- Calculate the moles of KOH using proportions
56 g of KOH ---------------- 1 mol
4 g of KOH ---------------- x
x = (4 x 1) / 56
x = 0.071 moles
2.- Substitute the values in the formula to find Molarity
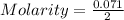
3.- Simplification and result
Molarity = 0.036 M