Answer:
The answer to your question is 0.033 M
Step-by-step explanation:
Data
moles of sucrose = 0.01
Molarity = ?
Volume = 300 ml
Formula
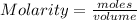
Process
1.- Convert volume to liters using proportions
1000 ml --------------------- 1 liter
300 ml ------------------- x
x = (300 x 1) / 1000
x = 300 / 1000
x = 0.3 liters
2.- Substitute values in the formula
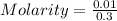
3.- Simplification and result
Molarity = 0.033