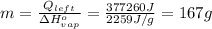Answer:
167 g
Step-by-step explanation:
We would firstly need to understand the phase changes happening during this process:
- the sample of water is heated until its boiling point firstly: we'll calculate the amount of heat required to reach the boiling point. This will be done using the specific heat capacity of water by
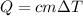 ;
; - the remaining amount of heat will be used to evaporate some mass of water.
Firstly, the normal boiling point of water is
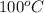 . Find the amount of heat required to heat the sample of water:
. Find the amount of heat required to heat the sample of water:
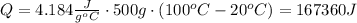
Now, find the amount of heat remaining:
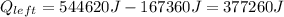
Let's use the equation for heat needed to evaporate water:
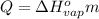
Here the enthalpy of vaporization is:
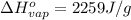
Use the amount of heat left to solve for the mass evaporated: